
A great outcome is one that lasts1.

A great outcome is one that lasts1.
Hydrus® Microstent:
Leading the Way For Evidence-Based Outcomes
In the 2020 American Academy of Ophthalmology (AAO) Primary Open-Angle Glaucoma Preferred Practice Pattern guidelines, the Hydrus® Microstent received a data rating of “moderate quality, strong recommendation”; the highest rating given among all MIGS.3*


59% of Hydrus® Microstent patients remained medication-free at 5 years12
*n=308


55% relative reduction in incisional SSI† for Hydrus® Microstent patients compared to cataract surgery alone (2.4% in Hydrus® vs. 5.3% in cataract surgery only)


Outstanding long-term safety at 60 months with SAE‡ rates comparable to cataract surgery alone1,2
†Secondary Surgical Intervention (SSI) includes trabeculectomy, tube shunt, gel stent, ECP/TSCP, non-penetrating; (9/369 Hydrus® and 10/187 CS)
‡SAE= Serious Adverse Event; (13/369 (3.5%) in Hydrus® eyes vs. 8/187 (4.3%) in the control eyes)
Hydrus® Microstent provides confident and efficient microstent delivery
Hydrus® Microstent’s intuitive delivery system allows for a straightforward procedure with immediate visual confirmation of successful implantation.
An Innovative Design and a Unique Mechanism of Action
Roughly the size of an eyelash, the Hydrus® Microstent is a revolutionary canal-based MIGS device for adult patients with mild to moderate primary open-angle glaucoma.
The device is made of nitinol, which is highly flexible and biocompatible.4
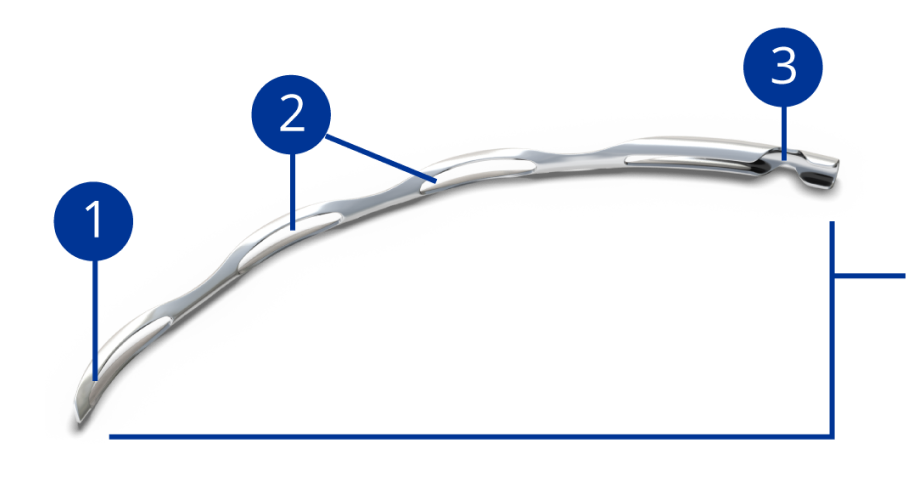
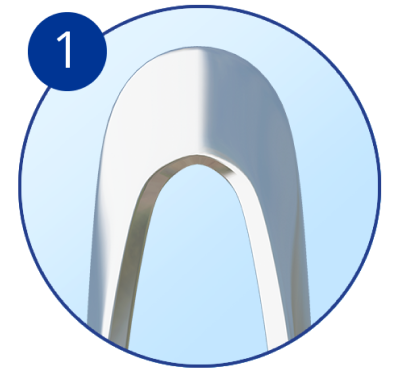

Rounded distal tip for a smooth passage into Schlemm’s canal


Open-window scaffold design provides outflow pathways for aqueous
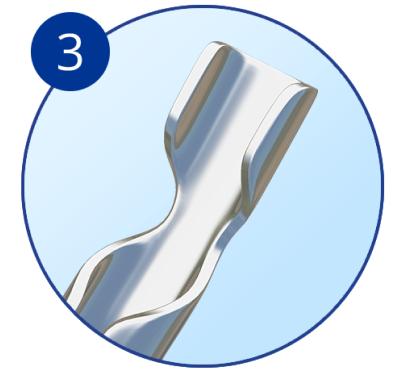

Aqueous inlet facilitates flow from anterior chamber to Schlemm’s canal
| |
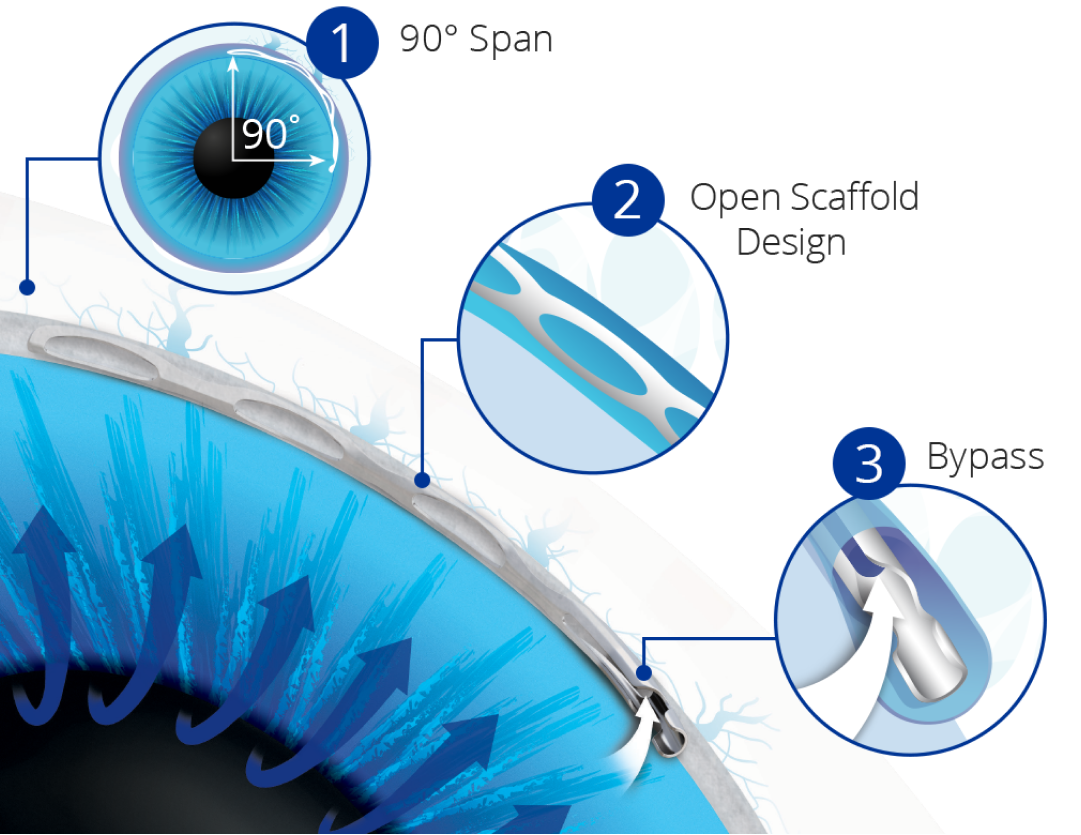
90° Span:The only MIGS implant to span approximately 90° of Schlemm’s canal, ensuring access to collector channels in the nasal region.
|
| |
Open Scaffold Design:The first MIGS device to precisely dilate and scaffold Schlemm’s canal, gently expanding the cross sectional area without obstructing outflow access to collector channel ostia.4
|
| |
Bypass:The Hydrus® Microstent bypasses the trabecular meshwork to restore flow of aqueous from the anterior chamber through the inlet of the microstent into Schlemm’s canal.
|
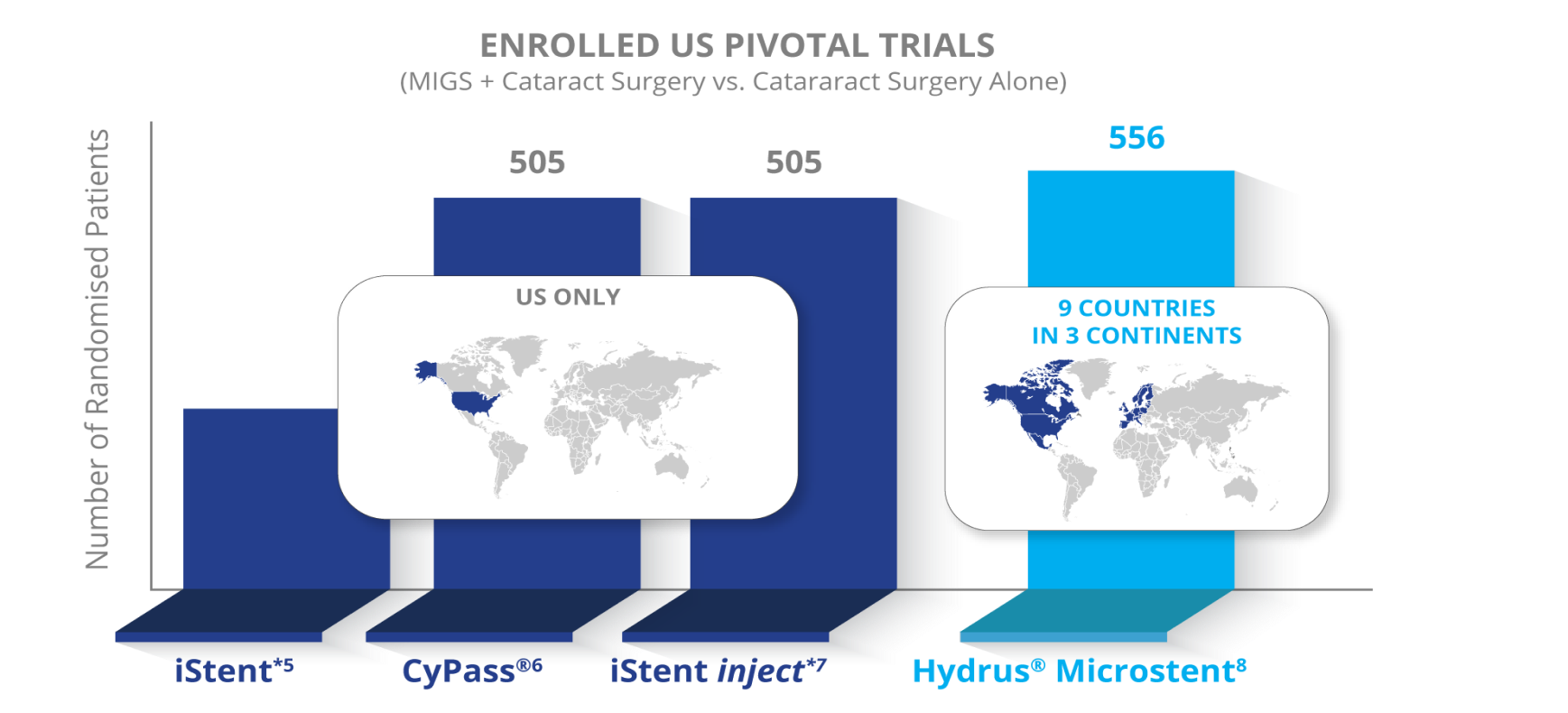
Gold Standard Data from a Randomized Controlled Pivotal Trial
The HORIZON Study
The largest prospective, randomised, controlled MIGS pivotal trial to date with 556 patients at 38 centers in 9 countries and five years of continuous follow up with 80% retention at year five.5-8
*Trademarks are the property of their respective owners.
Clinically proven to provide long-term IOP reduction9
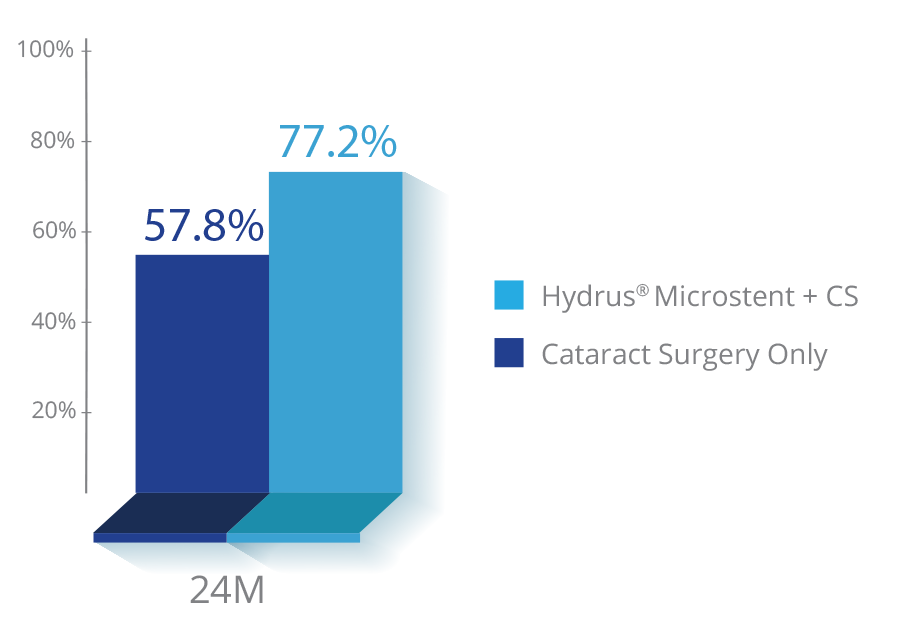


of eyes achieved a significant reduction in IOP at 2 years compared to 57.8% in cataract surgery-only patients9



greater mean IOP reduction at two years compared to cataract surgery alone9
*Statistically significant change; p<0.001


relative reduction
in incisional SSIs† at 5 years compared to cataract surgery alone1‡
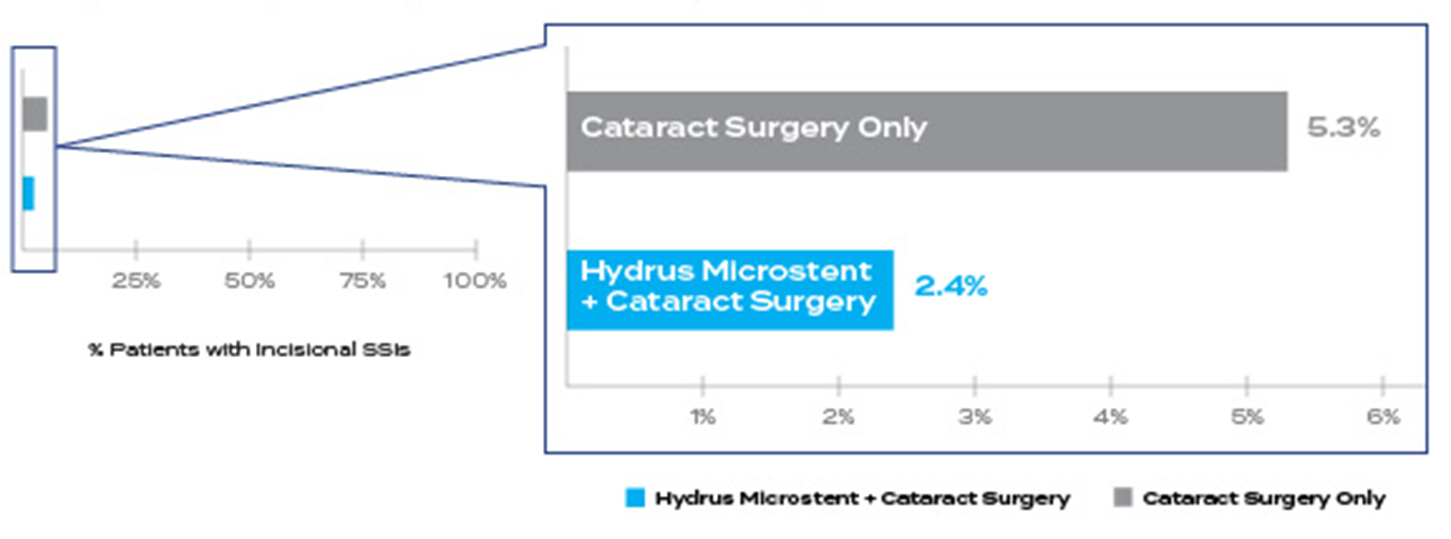

*5-year pivotal trial follow up included 308 patients in the Hydrus® group and 134 in the control
†Secondary Surgical Intervention (SSI) include trabeculectomy, tube shunt, gel stent, ECP/TSCP, non-penetrating; (9/369 Hydrus® and 10/187 CS)
Established long-term safety at 60-months with comparable SAE* rates reported vs cataract surgery alone1
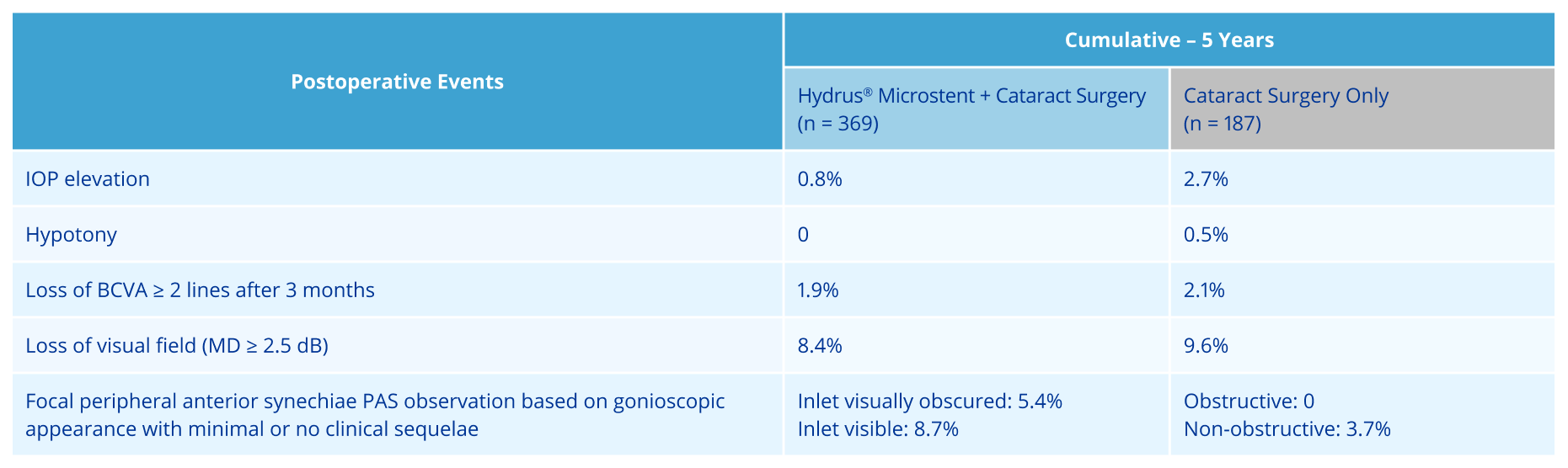
*SAE= Serious Adverse Event; (13/369 (3.5%) in Hydrus® eyes vs. 8/187 (4.3%) in the control eyes)
Using Hydrus® Microstent in Your Practice
Discover Hydrus® Microstent
Dr. Ike Ahmed Performs
Hydrus® Microstent Surgery
Hydrus® Microstent Clinical Studies
Presenting the 5-year results of the HORIZON trial comparing cataract surgery (CS) combined with an intracanalicular microstent with CS alone
NOTE: Ophthalmology articles are not open-source and require membership.
Comparing cataract surgery with implantation of a Schlemm canal microstent with cataract surgery alone for the reduction of intraocular pressure (IOP) and medication use after 24 months
NOTE: Ophthalmology articles are not open-source and require membership.
Determining improvement in outflow facility (C) in human anterior segments implanted with a novel Schlemm's canal scaffold or two trabecular micro-bypasses
Evaluating the efficacy and safety of ab interno trabecular bypass surgery with the Hydrus microstent in treating people with open angle glaucoma (OAG)
Instructions for Use (IFU)
Alcon Experience Academy
For relevant training content from industry thought leaders
IMPORTANT PRODUCT INFORMATION
IMPORTANT PRODUCT INFORMATION CAUTION: Federal law restricts this device to sale by or on the order of a physician. INDICATIONS FOR USE: The Hydrus Microstent is indicated for use in conjunction with cataract surgery for the reduction of intraocular pressure (IOP) in adult patients with mild to moderate primary open-angle glaucoma (POAG).
CONTRAINDICATIONS: The Hydrus Microstent is contraindicated under the following circumstances or conditions: (1) In eyes with angle closure glaucoma; and (2) In eyes with traumatic, malignant, uveitic, or neovascular glaucoma or discernible congenital anomalies of the anterior chamber (AC) angle.
WARNINGS: Clear media for adequate visualization is required. Conditions such as corneal haze, corneal opacity or other conditions may inhibit gonioscopic view of the intended implant location. Gonioscopy should be performed prior to surgery to exclude congenital anomalies of the angle, peripheral anterior synechiae (PAS), angle closure, rubeosis and any other angle abnormalities that could lead to improper placement of the stent and pose a hazard. The surgeon should monitor the patient postoperatively for proper maintenance of intraocular pressure. The surgeon should periodically monitor the status of the microstent with gonioscopy to assess for the development of PAS, obstruction of the inlet, migration, or device-iris or device-cornea touch. The Hydrus Microstent is intended for implantation in conjunction with cataract surgery, which may impact corneal health. Therefore, caution is indicated in eyes with evidence of corneal compromise or with risk factors for corneal compromise following cataract surgery. Prior to implantation, patients with history of allergic reactions to nitinol, nickel or titanium should be counseled on the materials contained in the device, as well as potential for allergy/hypersensitivity to these materials.
PRECAUTIONS: If excessive resistance is encountered during the insertion of the microstent at any time during the procedure, discontinue use of the device. The safety and effectiveness of use of more than a single Hydrus Microstent has not been established. The safety and effectiveness of the Hydrus Microstent has not been established as an alternative to the primary treatment of glaucoma with medications, in patients 21 years or younger, eyes with significant prior trauma, eyes with abnormal anterior segment, eyes with chronic inflammation, eyes with glaucoma associated with vascular disorders, eyes with preexisting pseudophakia, eyes with pseudoexfoliative or pigmentary glaucoma, and when implantation is without concomitant cataract surgery with IOL implantation. Please see a complete list of Precautions in the Instructions for use.
ADVERSE EVENTS: The most frequently reported finding in the randomized pivotal trial was peripheral anterior synechiae (PAS), with the cumulative rate at 5 years (14.6% vs 3.7% for cataract surgery alone). Other Hydrus postoperative adverse events reported at 5 years included partial or complete device obstruction (8.4%) and device malposition (1.4%). Additionally, there were no new reports of persistent anterior uveitis (2/369, 0.5% at 2 years) from 2 to 5 years postoperative. There were no reports of explanted Hydrus implants over the 5-year follow-up. For additional adverse event information, please refer to the Instructions for Use.
MRI INFORMATION: The Hydrus Microstent is MR-Conditional meaning that the device is safe for use in a specified MR environment under specified conditions. Please see the Instructions for Use for complete product information.
References
1. Ahmed IIK, et al. Long-term outcomes from the HORIZON randomized trial for Schlemm’s Canal microstent in combination cataract and glaucoma surgery. Ophthalmology. 2022;129(7):742-751.
2. NIH. Safety & effectiveness study of the Hydrus Microstent for lowering IOP in glaucoma patients undergoing cataract surgery (HORIZON). ClinTrials.gov (NCT01539239)
3. Gedde SJ, et al. Primary open-angle glaucoma preferred practice pattern. Ophthalmology 2020;128(1): 71-150.
4. Hydrus Microstent Instructions for Use. 2020.
5. US Food and Drug Administration. Summary of Safety and Effectiveness Data (SSED): Glaukos iStent® Trabecular Micro-Bypass Stent. https://www.accessdata.fda.gov/cdrh_docs/pdf8/P080030B.pdf. Published June 25, 2012.
6. US Food and Drug Administration. Summary of Safety and Effectiveness Data (SSED): CyPass System. https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150037B.pdf. Published July 29, 2016.
7. US Food and Drug Administration. Summary of Safety and Effectiveness Data (SSED): iStent inject Trabecular Micro-Bypass System. https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170043b.pdf. Published June 21, 2018.
8. US Food and Drug Administration. Summary of Safety and Effectiveness Data (SSED): CyPass System. Hydrus Microstent. https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170034B.pdf. Published August 10, 2018.
9. Samuelson TW, Chang DF, Marquis R, et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: The HORIZON Study. Ophthalmology. 2019;126:29-37.
10. Alcon Data on File, 2018.
11. Otarola F, Virgili G, Shah A, et al. Ab interno trabecular bypass surgery with Schlemm ́s canal microstent (Hydrus) for open angle glaucoma. Cochrane Database of Systematic Reviews. 2020;3:CD012740.
12. Alcon Data on File, 2024.




