Air Optix® Night & Day® Aqua
A flexible lens-wearing experience designed
for up to 30 nights of continuous wear*
*Extended wear for up to 30 continuous nights, as prescribed by an eye care professional.

Busy Patients Need Clear and Comfortable Vision, Whether It’s in the Middle of the Night…
…or first thing in the morning








AIR OPTIX® NIGHT & DAY® AQUA Can Help These Patients With SmartShield® Technology and Ultimate Breathability2
AIR OPTIX™ NIGHT & DAY™ AQUA contact lenses are FDA approved for up to 30 days and nights of continuous wear.


SmartShield® Technology
Delivers a protective layer of moisture to help shield lenses from irritating deposits3,4,5


Highest Breathability6*
Oxygen continuously flows through the lens for white, healthy looking eyes2


A Winning Combination
Your patients can wake up to comfortable, clear vision
*Dk/t = 175 @ -3.00D. Other factors may impact eye health.
Unique SmartShield® Technology Helps Offer Lasting Comfort, And Deposit Resistance4,5
Air Optix® Night & Day® Aqua contact lenses are designed with SmartShield® Technology, to protect the lens, retain moisture, and resist deposits.3,4,5 This means lenses stay comfortable, day after day.
Look Closer at a Contact Lens Surface Like no Other




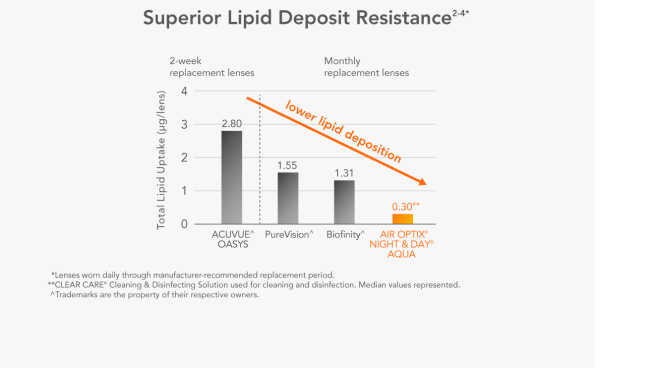
Superior Lipid Deposit Resistance4,5*
Lenses worn daily through manufacturer-recommended replacement period. CLEAR CARE® Cleaning & Disinfecting Solution used for cleaning and disinfection. Median values represented.
Superior Wettability3*
In vitro measurement of unworn lenses, median values represented, n=20.
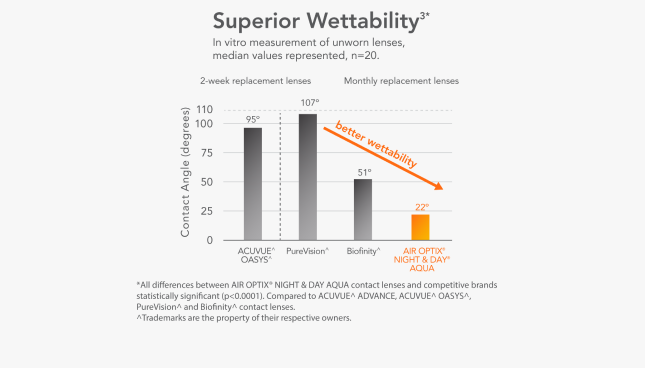
*All differences between AIR OPTIX® NIGHT & DAY® AQUA® contact lenses and competitive brands statistically significant (p<0.0001).
Compared to Biofinity,* PureVision,* ACUVUE* OASYS,* ACUVUE* ADVANCE *.
To Help Your Patients Get the Most From Their Contact Lenses, Recommend CLEAR CARE® PLUS and OPTI-FREE® Puremoist®

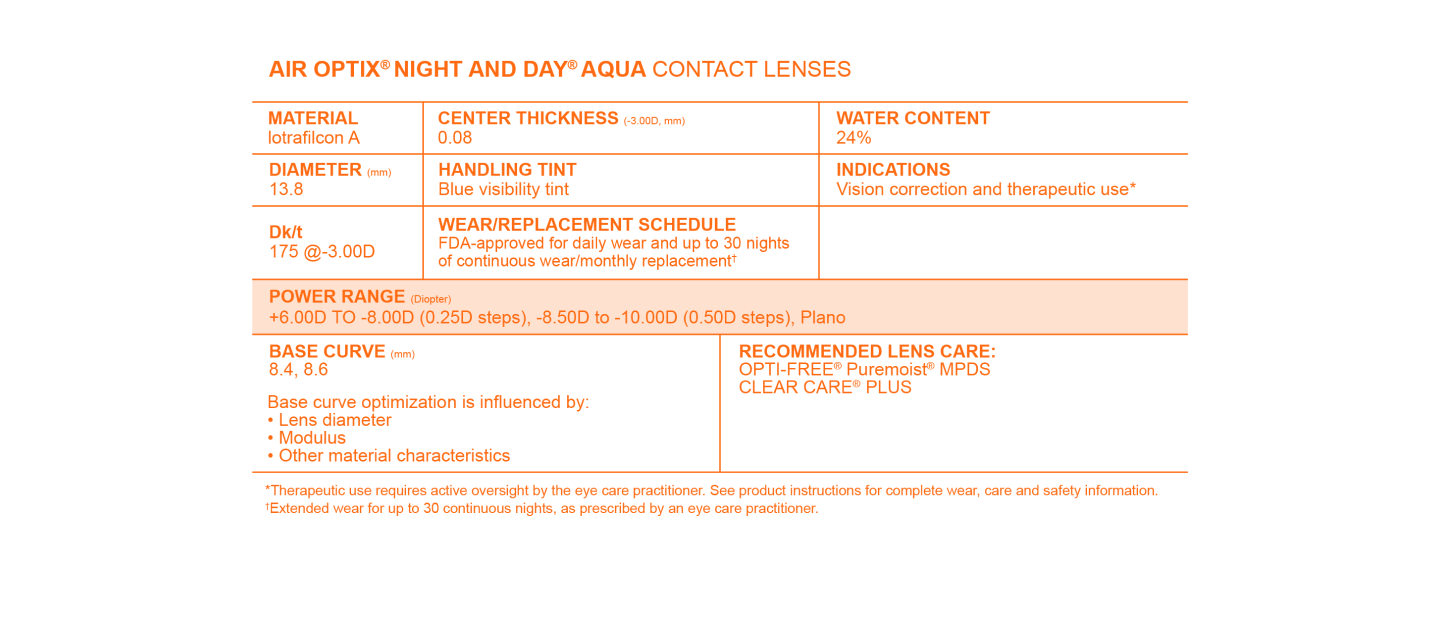
AIR OPTIX® NIGHT & DAY® AQUA Contact Lenses Product Information


AIR OPTIX® plus HydraGlyde® Multifocal
Delivers outstanding comfort*, long-lasting lens surface moisture4 and seamless vision at all distances5
*Based on clinical studies with Air Optix® Aqua Multifocal contact lenses


AIR OPTIX® plus HydraGlyde® for Astigmatism
Designed to provide comfortable*, clear vision
*Based on clinical studies with Air Optix® for Astigmatism contact lenses
See product instructions for complete wear, care and safety information. ![]()
Important information for AIR OPTIX® NIGHT & DAY® AQUA (lotrafilcon A) contact lenses: Indicated for vision correction for daily wear (worn only while awake) or extended wear (worn while awake and asleep) for up to 30 nights.
Relevant Warnings: A corneal ulcer may develop rapidly and cause eye pain, redness or blurry vision as it progresses. If left untreated, a scar, and in rare cases loss of vision, may result. The risk of serious problems is greater for extended wear vs. daily wear and smoking increases this risk. A one-year post-market study found 0.18% (18 out of 10,000) of wearers developed a severe corneal infection, with 0.04% (4 out of 10,000) of wearers experiencing a permanent reduction in vision by two or more rows of letters on an eye chart.
Relevant Precautions: Not everyone can wear for 30 nights. Approximately 80% of wearers can wear the lenses for extended wear. About two-thirds of wearers achieve the full 30 nights continuous wear.
Side Effects: In clinical trials, approximately 3-5% of wearers experience at least one episode of infiltrative keratitis, a localized inflammation of the cornea which may be accompanied by mild to severe pain and may require the use of antibiotic eye drops for up to one week. Other less serious side effects were conjunctivitis, lid irritation or lens discomfort including dryness, mild burning or stinging.
Contraindications: Contact lenses should not be worn if you have: eye infection or inflammation (redness and/or swelling); eye disease, injury or dryness that interferes with contact lens wear; systemic disease that may be affected by or impact lens wear; certain allergic conditions or using certain medications (ex. some eye medications).
Additional Information: Lenses should be replaced every month. If removed before then, lenses should be cleaned and disinfected before wearing again. Always follow the eye care professional’s recommended lens wear, care and replacement schedule. Consult package insert for complete information, available without charge by calling (800) 241-5999 or go to myalcon.com
Important information for AIR OPTIX® plus HydraGlyde® Astigmatism (lotrafilcon B) contact lenses: For daily wear or extended wear up to 6 nights for near/far-sightedness. Risk of serious eye problems (i.e., corneal ulcer) is greater for extended wear. In rare cases, loss of vision may result. Side effects like discomfort, mild burning or stinging may occur.
Important information for AIR OPTIX® plus HydraGlyde® Multifocal (lotrafilcon B) contact lenses: For daily wear or extended wear up to 6 nights for near/far-sightedness. Risk of serious eye problems (i.e., corneal ulcer) is greater for extended wear. In rare cases, loss of vision may result. Side effects like discomfort, mild burning or stinging may occur.
ˆTrademarks are the property of their respective owners.
- Alcon data on file, 2012.
- Based on the ratio of lens oxygen transmissibilities; Alcon data on file, 2009, 2010.
- Alcon data on file, 2009.
- Nash W, Gabriel M, Mowrey-McKee M. A comparison of various silicone hydrogel lenses; lipid and protein deposition as a result of daily wear. Optom Vis Sci. 2010;87: E-abstract 105110.
- Alcon data on file, 2017.
- Based on published manufacturer-provided DK/T values in Tylers Quarterly, 2022
- Schein O, McNally J, Katz J, Chalmers R. The incidence of microbial keratitis among wearers of a 30-day silicone hydrogel extended-wear contact lens. Ophthalmology. 2005;112(12):2172-79.


