MyAlcon | Sweden
This page is available in English. Please click below for other locations.
AcrySof™ IQ Monofocal and AcrySof™ IQ Toric IOLs
The trusted IOL that gives you complete confidence
AcrySof™ IQ Monofocal and AcrySof™ IQ Toric IOLs
The trusted IOL that gives you complete confidence
AcrySof™ IQ Monofocal IOLs Give You Complete Confidence You Made The Right Choice
Low PCO and Nd: YAG Rates1-8
Helps free-up capacity by helping to reduce secondary treatments
Excellent Quality of Vision1,9-12,16-23
Proven long-lasting visual outcomes
Trusted by Surgeons24,25
Over 125 million implants worldwide

Extensive Research Proves AcrySof™ Has Low Nd:YAG Rates at 3 and 5 years2*
Unprecedented data demonstrates AcrySof™ reduces Nd: YAG treatment4-8
Multiple studies conducted across various countries reveal why AcrySof™ IQ should be the right IOL choice.


Reduced development of PCO
AcrySof™ IQ material promotes clear capsules and reduces secondary treatments:1,3
- Unique AcrySof™ IQ hydrophobic acrylic material provides the highest bonding with the capsule compared to competitor IOLs.*
- Proprietary edge design helps to block cell migration between the optic and the capsular bag.
*compared to PMMA, HOYA, and TECNIS IOLs, n=32 IOLs per group, P<0.001

Excellent Quality of Vision
AcrySof™ IQ BioMechanics provide excellent refractive outcomes

*Trademarks are the property of their respective owners.
Available in two filtering properties that mimic the natural human lens1,16,18
UV and Blue light filter
Proprietary chromophore that can help filter harmful light sources, similar to the healthy human lens1,18
The AcrySof™ IQ blue light filter can provide patients with a greater tolerance for extreme bright light as well as faster visual recovery after high bright light exposure1,16-19


UV filter
AcrySof™ provides amongst the highest level of UV radiation filter. Some IOLs allow parts of UVA radiation to pass through the IOL and to the retina.1,12-15
*Other IOLs include J&J Tecnis†, and B&L Envista†. See UV filter chart below for further details.
†Trademarks are the property of their respective owners.
AcrySof™ blue light filter helps patients manage conditions they encounter every day.1,16-19*†
- Greater tolerance for extreme bright light16,18,19
- Faster visual recovery after high bright light exposure16,18,19
Improved Performance in Challenging Conditions*

*A subset of patients (n = 44) underwent testing in a validated night driving simulator. Patients were tested monocularly under conditions which simulate city and rural settings under normal, glare and fog conditions.
†AcrySof™ IQ IOL patients had an average increase of 40+ metres feet versus the control lens in which to stop after identifying a warning sign in a rural setting under fog conditions at 88 km/h.
High Level of UV Protection1
AcrySof™ provides amongst the highest level of UV radiation filter close to the 400nm cutoff1,12-15,23

Fully usable optic helps reduce visual disturbances
The AcrySof™ IQ aspheric optic surface is designed to reduce edge glare.1


AcrySof™ (SN60WF)
6 mm fully usable aspheric optic1,20


TECNIS* 1-Piece (ZCB00)
4.9 mm limited usable aspheric optic13,20


enVista* (MX60)
4.9 mm limited usable aspheric optic15,20
*Trademarks are the property of their respective owners
Trusted by Surgeons Worldwide
AcrySof™ IQ features proven technology for a confidence that has been truly earned.

RANKED #1
in surgical IOLs24


125+ MILLION
AcrySof™ IOLs implanted worldwide25


25+ YEARS
proven safety and effectiveness

BACKED BY
unprecedented clinical data
AcrySof™ IQ is available in the UltraSert™ Preloaded Delivery System

Proven Stability and Excellent Performance with AcrySof™ IQ Toric IOL

AcrySof™ IQ Toric IOLs had significantly lower incidence of repositioning compared to other IOLs27*
Based on Retrospective comparison to TECNIS* Toric and HOYA 355 Toric Intraocular lens, (n=9430 eyes, p<0.0001)
Alcon Online Toric IOL Calculator features the Barrett Algorithm

AcrySof™ IQ and Toric IOL Clinical Studies
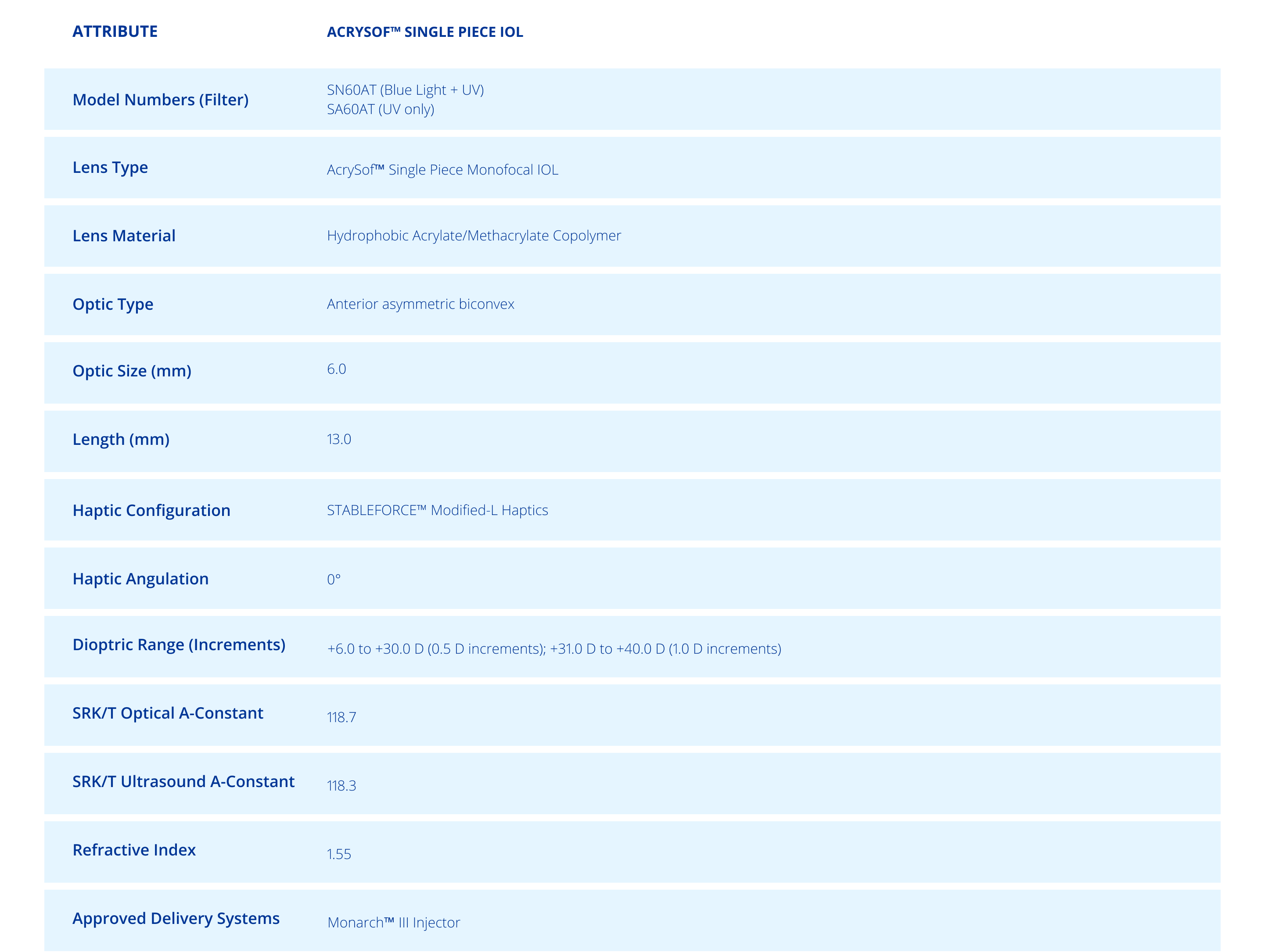
Technical Specifications1
ACRYSOF™ IQ MONOFOCAL

ACRYSOF™ IQ TORIC

ACRYSOF™ SINGLE PIECE
ACRYSOF™ MULTIPIECE

Instructions for Use (IFU)
For a full list of indications, contraindications and warnings, please visit ifu.alcon.com and refer to the relevant product’s instructions for use.
Alcon Experience Academy
For relevant training content from industry thought leaders
References:
1. AcrySof™ IQ Directions For Use.
2. RCOphth National Ophthalmology Database Audit Feasibility Study of Post-cataract Posterior Capsule Opacification https://www.nodaudit.org.uk/u/docs/20/rijbxkcubs/RCOphth%20NOD%20PCO%20Report%202021.pdf Accessed 14 July 2021.
3. Ong M, Wang L, Karakelle M. Fibronectin adhesive properties of various intraocular lens materials. Alcon Laboratories, Fort Worth, TX, USA. ARVO 2013.
4. Ursell PG, Dhariwal M, Majirska K, Ender F, Kalson-Ray S, Venerus A, Miglio C, Bouchet C. Three-year incidence of Nd:YAG capsulotomy and posterior capsule opacification and its relationship to monofocal acrylic IOL biomaterial: a UK Real World Evidence study. Eye (Lond). 2018 Oct;32(10):1579-1589.
5. Belda J, Placeres J, Elvira J, O’Boyle D, Puig X, Vives CP, et al. Is Nd:YAG capsulotomy incidence influenced by IOL biomaterial? Real-word evidence from Spain at 3 & 5 years after cataract surgery. Free Paper presented at the European Society of Cataract and Refractive Surgeons annual meeting; October 2020; Virtual Congress.
6. Lindholm JM, Laine I, Tuuminen R. Five-Year Cumulative Incidence and Risk Factors of Nd:YAG Capsulotomy in 10 044 Hydrophobic Acrylic 1-Piece and 3-Piece Intraocular Lenses. Am J Ophthalmol. 2019 Apr;200:218-223.
7. Thom H, Ender F, Samavedam S, Perez Vivez C, Gupta S, Dhariwal M, de Haan J, O'Boyle D. Effect of AcrySof versus other intraocular lens properties on the risk of Nd:YAG capsulotomy after cataract surgery: A systematic literature review and network meta-analysis. PLoS One. 2019 Aug 19;14(8):e0220498.
8. Ursell PG, Dhariwal M, O'Boyle D, Khan J, Venerus A. 5 year incidence of YAG capsulotomy and PCO after cataract surgery with single-piece monofocal intraocular lenses: a real-world evidence study of 20,763 eyes. Eye (Lond). 2020 May;34(5):960-968.
9. Wirtitsch MG, Findl O, Menapace R, Kriechbaum K, Koeppl C, Buehl W, Drexler W. Effect of haptic design on change in axial lens position after cataract surgery. J Cataract Refract Surg. 2004 Jan;30(1):45-51.
10. Nejima R, Miyai T, Kataoka Y, Miyata K, Honbou M, Tokunaga T, Kawana K, Kiuchi T, Oshika T. Prospective intrapatient comparison of 6.0-millimeter optic single-piece and 3-piece hydrophobic acrylic foldable intraocular lenses. Ophthalmology. 2006 Apr;113(4):585-90.
11. Kohnen T, Klaproth OK. Incision sizes before and after implantation of SN60WF intraocular lenses using the Monarch injector system with C and D cartridges. J Cataract Refract Surg. 2008 Oct;34(10):1748-53.
12. Mainster MA. Spectral transmittance of intraocular lenses and retinal damage from intense light sources. Am J Ophthalmol. 1978 Feb;85(2):167-70.
13. Tecnis 1-piece IOL with the Tecnis iTec Preloaded Delivery System Directions For Use. Johnson & Johnson Vision.
14. HOYA NY-60 Direction For Use. HOYA Corporation.
15. EnVista hydrophobic acrylic intraocular lens Directions For Use. Bausch & Lomb.
16. Gray R, Perkins SA, Suryakumar R, Neuman B, Maxwell WA. Reduced effect of glare disability on driving performance in patients with blue light-filtering intraocular lenses. J Cataract Refract Surg. 2011 Jan;37(1):38-44
17. Wei X, She C, Chen D, Yan F, Zeng J, Zeng L, Wang L. Blue-light-blocking intraocular lens implantation improves the sleep quality of cataract patients. J Clin Sleep Med. 2013 Aug 15;9(8):741-5.
18. Landers JA, Tamblyn D, Perriam D. Effect of a blue-light-blocking intraocular lens on the quality of sleep. J Cataract Refract Surg. 2009 Jan;35(1):83-8.
19. Hammond BR Jr, Renzi LM, Sachak S, Brint SF. Contralateral comparison of blue-filtering and non-blue-filtering intraocular lenses: glare disability, heterochromatic contrast, and photostress recovery. Clin Ophthalmol. 2010;4:1465-1473.
20. Alcon Data on File, 2017. TDOC-0053803 - Imaging of the Usable Optic Diameter of Clareon SY60WF, Tecnis ZCB00, and enVista MX60 Intraocular Lenses.
21. Lane, S, Collins, S, Das, K et al. Evaluation of intraocular lens mechanical stability. J Cataract Refract Surg. 2019;45(4):501-506.
22. Alcon Data on File, 2017. TDOC-0054422 - Axial Displacement Images at 9mm Compression.
23. García-Domene MC, Pérez-Vives C, Peris-Martínez C, Artigas JM. Comparison of the Ultraviolet Light Filtering across Different Intraocular Lenses. Optom Vis Sci. 2018 Dec;95(12):1129-1134.
24. Based on 2020 sales reported in Market Scope, GfK, Nielsen, IQVIA, Euromonitor, and Alcon internal estimates.
25. Alcon sales data since 1993.
26. Oshika T, Fujita Y, Hirota A, et al. Comparison of incidence repositioning surgery to correct misalignment with three toric intraocular lenses. Eur J Ophthalmol. 2019. doi:10.1177/1120672119834469.
27. Lee BS, Chang DF. Comparison of the rotational stability of two toric intraocular lenses in 1273 consecutive eyes. Ophthalmology. 2018;125(9):1325-1331.
Medical Devices manufactured by Alcon comply with all applicable laws and regulations. For indications, contraindications, warnings and serious incidents please refer to the relevant product’s direction for use or operator manuals.
