MyAlcon | Sweden
This page is available in English. Please click below for other locations.
UltraSert™ Preloaded Delivery System
UltraSert™ Preloaded Delivery System
Gain consistency in IOL delivery, injection control and enhance the efficiency of your OR1
NORD-ULS-2300002
Consistency in IOL Delivery for Reduced Intraoperative Variability1-3

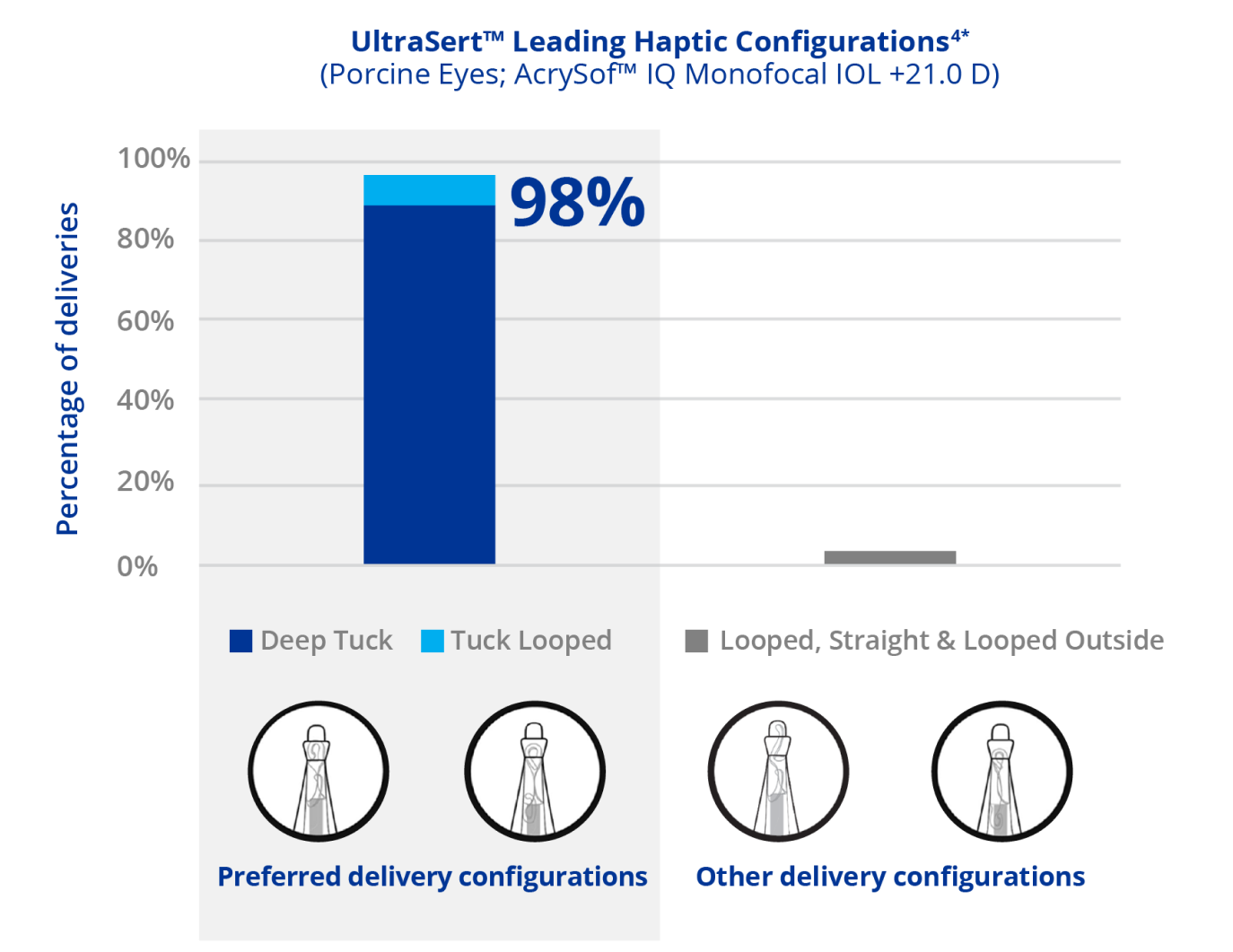
*Observations during porcine cadaver eye study to evaluate consistency of leading haptic configuration using permissible delivery configurations identified in the UltraSert™ Directions for Use. Study conducted using UltraSert™ optimized with 3 mm (extended) tip and inner lumen adjustment (n=184-187). VISCOAT™ OVD was used at operating room temperatures (approximately 65 to 73 degrees).
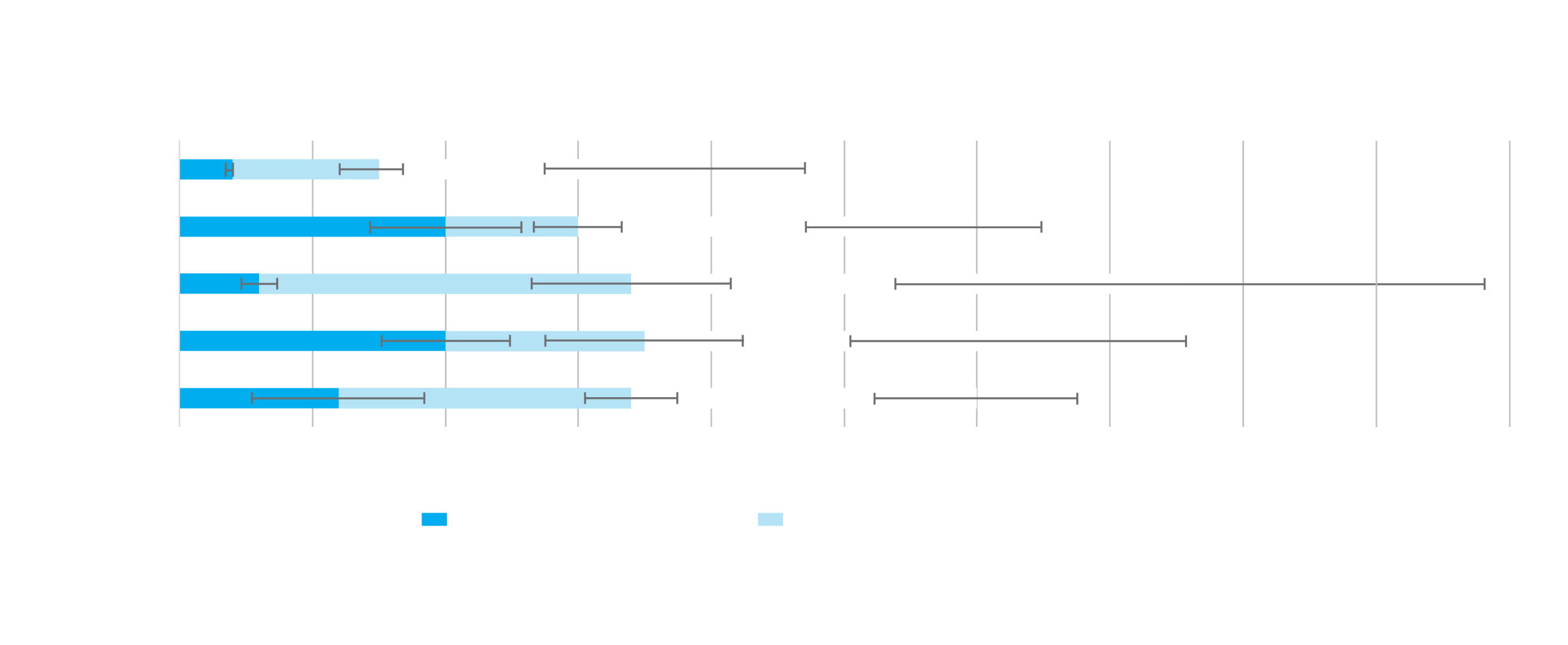
UltraSert™ helps minimise wound stretch compared to other delivery devices1,3,5

*Before IOL implantation, corneal incisions were made (2.2 mm knife for IOL implantation with UltraSert™, iTec† and iSert† 250/251; 2.4 mm knife for IOL implantation with Monarch™ III) and initial incision size was measured and documented. Post-IOL implantation incision size was measured and documented via the same process. UltraSert® (n=19) presented with a significantly smaller mean corneal incision size after IOL implantation compared with iTec† (n=26), iSert† 250/251 (n=26) and Monarch™ III (n=28). For each, p < 0.001. Testing completed with first-generation UltraSert™ (2 mm nozzle tip). †Trademarks are the property of their respective owners
Enhanced Injection Control for Smooth IOL Delivery1
UltraSert™ features the spring-controlled TensionGlide™ Plunger for smooth IOL deployment.1,6
TensionGlide™ maintains injection force stability6
NORD-ULS-2300003
TensionGlide™ mitigates the risk of sudden forceful IOL ejection, offering the ideal amount of resistance6,7

Efficiency and Enhanced Patient Throughput8,9
UltraSert™ helps streamline patient throughput with a low average preparation time.8,9
UltraSert™ is ready to implant in 3 simple steps10

NORD-ULS-2300004
UltraSert™ is Preloaded with AcrySof™ IQ IOL10
The trusted IOL that gives you complete confidence

Clinical Studies
Technical Specifications1

Instructions for Use (IFU)
For a full list of indications, contraindications and warnings, please visit ifu.alcon.com and refer to the relevant product’s instructions for use.
Alcon Experience Academy
For relevant training content from industry thought leaders
References:
1. Alcon Data on File, REF-02270, 2015.
2. Wang L, Wolfe P, Chernosky A, Paliwal S, Tjia K, Lane S. In vitro delivery performance assessment of a new preloaded intraocular lens delivery system. J Cataract Refract Surg. 2016;42(12):1814–1820.
3. Mendicute J, Amzallag T, Wang L, Martinez AA. Comparison of incision size and intraocular lens performance after implantation with three preloaded systems and one manual delivery system. Clin Ophthalmol. 2018;12:1495–1503.
4. Alcon Data on File, REF-04197, 2019.
5. Amzallag T, Mendicute J, Martinez A. Multicenter clinical assessment of a pre-loaded IOL delivery system. Paper presented at ASCRS Congress; May 5-9, 2017; Los Angeles, CA.
6. Alcon Data on File, REF-02260, 2016.
7. Alcon Data on File, REF-07653, 2015.
8. Nanavaty MA, Kubrak-Kisza M. Evaluation of preloaded intraocular lens injection systems: Ex vivo study. J Cataract Refract Surg. 2017 Apr;43(4):558-563.
9. Mendicute J, Pablo L, Vélasque L, Martinez A, Asmar J, Schweitzer C. Multicenter evaluation of time, operational and economic efficiencies of a new pre-loaded intraocular lens delivery system vs. manual intraocular lens delivery. Clin Ophthalmol. 2021:15;591-599.
10. AcrySof IQ with UltraSert Product Information, 2017.
Medical Devices manufactured by Alcon comply with all applicable laws and regulations. For indications, contraindications, warnings and serious incidents please refer to the relevant product’s direction for use or operator manuals.
