1 drop.
8 hours
dry eye relief.2
Our most advanced dry eye solution, preservative-free

SYSTANE® COMPLETE Preservative-Free
See how the technology and science of SYSTANE® COMPLETE PF supports all layers of the tear film and protects against tear evaporation3,4

An aging population, pollution, and an increase in the use of digital devices all contribute to the rapidly growing dry eye market5,6
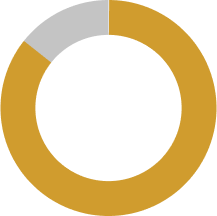

86% of dry eye sufferers have clinical signs of Meibomian Gland Dysfunction and may benefit from a drop like SYSTANE® COMPLETE PF7
SYSTANE® COMPLETE Preservative-Free gives your patients what they want
Effective | Provides immediate and lasting symptom relief


*Based on 45.5% of baseline patients, who reported moderate to severe symptoms; n=61
Ergonomic | New multi-dose bottle is designed for easier handling and less drop spillage.*
86%
of patients prefer MDPF packaging
over unit-dose vials8**
94%
of ECPs said the bottle
used for SYSTANE® MDPF products
is easy and intuitive8†
*vs. usage of unit-dose vials
**n=155
†n=17

Economical

Helps with a recommendation barrier of preservative-free artificial tears based on cost.9
*Compared to SYSTANE® HYDRATION PF UD vials.

Environment | Multi-dose bottle reduces packaging and plastic waste
Saves nearly two pounds of plastic per customer per year10*
- One multi-dose bottle replaces 120 unit-dose vials10**
- ~96% reduction in plastic vs. unit-dose preservative-free vials10*
*Based on two drops per eye of SYSTANE® COMPLETE Preservative-Free per day and two unit-dose vials of dry eye drops per day.
**Calculation based on comparison of SYSTANE® COMPLETE PF vs. SYSTANE® HYDRATION UD; 1 drop per eye per dose.

Explore the rest of the SYSTANE® portfolio
1. Based on IQVIA ProVoice Survey of Eye Care Professionals 12 months ending December 31, 2021.
2. Silverstein S, Yeu E, Tauber J, et al. Symptom Relief Following a Single Dose of Propylene Glycol-Hydroxypropyl Guar Nanoemulsion in Patients with Dry Eye Disease: A Phase IV, Multicenter Trial. Clin Ophthalmol. 2020;14:3167-3177.
3. Rangarajan R, Ketelson H. Preclinical evaluation of a new hydroxypropyl-guar phospholipid nanoemulsion-based artificial tear formulation in models of corneal epithelium. J Ocul Pharmacol Ther. 2019;35(1):32-37.
4. Ketelson H, Rangarajan R. Pre-clinical evaluation of a novel phospholipid nanoemulsion based lubricant eye drops. Poster presented at ARVO 2017, Baltimore, MD.
5. MarketScope LLC. 2020 Dry Eye Products Report: A Global Market Analysis for 2019 to 2025. St. Louis, MO: MarketScope LLC; 2020.
6. Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am J Ophthalmol. 2017;182:90-98.
7. Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31:472-478.
8. Based on a survey of 155 patients and 17 eye care professionals in Canada; patients used SYSTANE® HYDRATION MDPF for one month before taking survey; Alcon data on file, 2021.
9. Alcon data on file, 2021.
10. Alcon data on file, 2021.
©2022 Alcon Inc. US-SYX-2200002


