Clareon® Vivity® Extended Vision IOL
Expand what’s possible: the first and only non-diffractive presbyopia-mitigating IOL with exceptional clarity1,2*
Clareon® Vivity® Extended Vision IOL
Expand what’s possible: the first and only non-diffractive presbyopia-mitigating IOL with exceptional clarity1,2*
Provide patients with monofocal-quality distance with excellent intermediate and functional near vision.1† Vivity® expands visual possibilities.
By harnessing the power of non-diffractive X-WAVE™ Technology, the Clareon® Vivity® IOL helps your patients take advantage of enhanced vision where they need it most.


Monofocal-quality
distance vision
Binocular Mean Uncorrected Distance Visual Acuity1†‡
20/20


Excellent intermediate
vision
Binocular Mean Uncorrected Intermediate Visual Acuity (26 in)1†
>20/25


Functional near
vision
Binocular Mean Uncorrected Near Visual Acuity (16 in)1†‡
20/32
Low levels of patient reported visual disturbances1†§‖
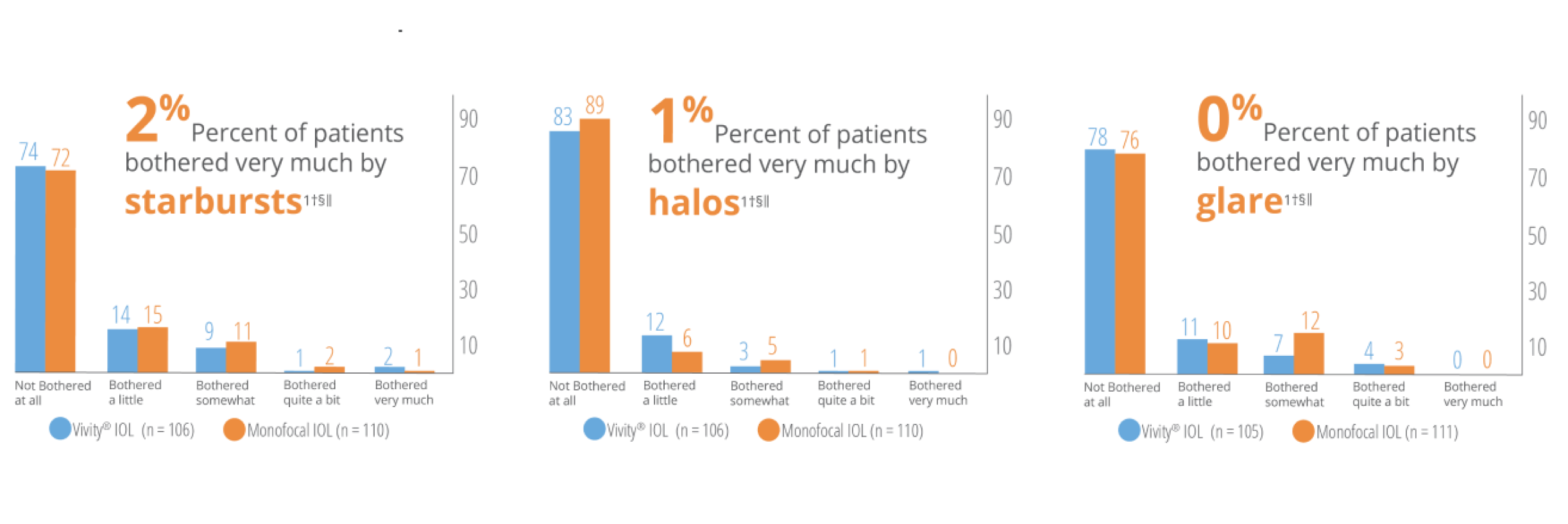
* Based on in vitro examinations of glistenings, surface haze and SSNGs.
† Results from a prospective, randomized, parallel group, subject- and assessor-masked, multisite trial of 107 subjects bilaterally implanted with the AcrySof® IQ Vivity® IOL and 113 with the AcrySof® IQ IOL with 6 months follow-up.
‡ Snellen VA was converted from logMAR VA. A Snellen notation of 20/20-2 or better indicates a logMAR VA of 0.04 or better, which means 3 or more of the 5 ETDRS chart letters in the line were identified correctly.
§ Assessed using QUVID questionnaire.
‖ AcrySof® IQ Vivity® was tested. AcrySof® IQ Vivity® and Clareon® Vivity® are optically equivalent.
Hear what surgeons are saying about the Clareon® Vivity® IOL
Explore additional resources for the Clareon® Monofocal IOL
Non-diffractive X-WAVE™ Technology
This proprietary technology features two smooth surface transition elements that simultaneously stretch and shift light without splitting it.3

Strong patient satisfaction demonstrated in clinical studies


90%
of patients were satisfied with their results and would get the Clareon® Vivity® IOL again.4†||¶


93%
of patients would recommend the Clareon® Vivity® IOL to a friend or family member.4†||¶
† Results from a prospective, randomized, parallel group, subject- and assessor-masked, multisite trial of 107 subjects bilaterally implanted with the AcrySof® IQ Vivity® IOL and 113 with the AcrySof® IQ IOL with 6 months follow-up.
‖ AcrySof® IQ Vivity® was tested. AcrySof® IQ Vivity® and Clareon® Vivity® are optically equivalent.
¶ Response to the following question in IOLSAT questionnaire at 6 months post-op: “Given your vision today, if you had to do it all over, would you have the same lens implanted again?”
§ Assessed using QUVID questionnaire.
‖ AcrySof® IQ Vivity® was tested. AcrySof® IQ Vivity® and Clareon® Vivity® are optically equivalent.
Hear about the impact the Clareon® Vivity® IOL is having on patients
Share our product brochure with your patients to communicate the Clareon® difference


Available on Clareon®: Alcon’s most advanced IOL biomaterial to date



IMPORTANT PRODUCT INFORMATION - CLAREON® ASPHERIC FAMILY OF HYDROPHOBIC ACRYLIC IOLS
CAUTION: Federal law restricts this device to sale by or on the order of a physician.
INDICATIONS: The Clareon® Vivity® Extended Vision Hydrophobic Posterior Chamber IOLs include Clareon® Vivity® and Clareon® Vivity® Toric IOLs and are indicated for primary implantation for the visual correction of aphakia in adult patients with <1.00 D of preoperative corneal astigmatism, in whom a cataractous lens has been removed by extracapsular cataract extraction. The lens mitigates the effects of presbyopia by providing an extended depth of focus. Compared to an aspheric monofocal IOL, the lens provides improved intermediate and near visual acuity, while maintaining comparable distance visual acuity. The Clareon® Vivity® IOL is intended for capsular bag placement only. In addition, the Clareon® Vivity® Toric IOL is indicated for the reduction of residual refractive astigmatism in adult patients with pre-existing corneal astigmatism.
WARNINGS / PRECAUTIONS: Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/benefit ratio before implanting a lens in a patient with any of the conditions described in the Directions for Use labeling. This lens should not be implanted if the posterior capsule is ruptured, if the zonules are damaged, or if a primary posterior capsulotomy is planned. Rotation can reduce astigmatic correction; if necessary, lens repositioning should occur as early as possible prior to lens encapsulation. Most patients implanted with the Clareon® Vivity® IOL are likely to experience significant loss of contrast sensitivity as compared to a monofocal IOL. Therefore, it is essential that prospective patients be fully informed of this risk before giving their consent for implantation of the Clareon® Vivity® IOL. In addition, patients should be warned that they will need to exercise caution when engaging in activities that require good vision in dimly lit environments, such as driving at night or in poor visibility conditions, especially in the presence of oncoming traffic. It is possible to experience very bothersome visual disturbances, significant enough that the patient could request explant of the IOL. In the parent AcrySof® IQ Vivity® IOL clinical study, 1% to 2% of AcrySof® IQ Vivity® IOL patients reported very bothersome starbursts, halos, blurred vision, or dark area visual disturbances; however, no explants were reported. Prior to surgery, physicians should provide prospective patients with a copy of the Patient Information Brochure available from Alcon informing them of possible risks and benefits associated with the Clareon® Vivity® IOLs.
ATTENTION: Reference the Directions for Use labeling for each IOL for a complete listing of indications, warnings and precautions.
REFERENCES:
- Clareon® Vivity® Extended Vision Hydrophobic IOL (CNWET0) Directions for Use – USA.
- Werner L, Thatthamla I, Ong M, et al. Evaluation of clarity characteristics in a new hydrophobic acrylic IOL. J Cataract Refract Surg. 2019;45:1490-1497.
- Alcon Data on File, 2019.
- Alcon Data on File, 2019.




