Ophthalmic Viscosurgical Devices (OVDs)
Alcon OVDs are trusted and consistent products designed for optimised protection of the cornea, with cohesive and dispersive properties and sufficient volume for every step of the procedure.
Ophthalmic Viscosurgical Devices (OVDs)
Alcon OVDs are trusted and consistent products designed for optimised protection of the cornea, with cohesive and dispersive properties and sufficient volume for every step of the procedure.
Chondroitin Sulphate Provides Enhanced Endothelial Coating1-4
Chondroitin sulphate is designed with a double negative charge and when combined with sodium hyaluronate, creates a triple negative charged product. This results in improved adhesion to positively charged endothelial tissue.1-4*
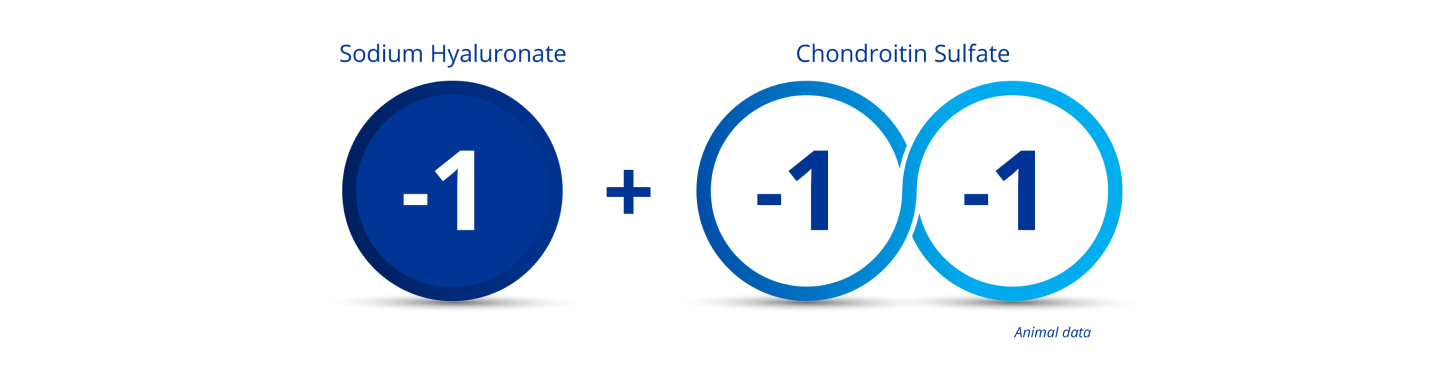
*Compared to only-Na-Hyaluronate OVDs
Viscoelastic products containing chondroitin sulphate improve OVD retention.2*
Chondroitin sulphate-containing viscoelastic products demonstrate excellent retention and coating of the endothelium under stress from high flow conditions. This leads to less endothelial cell loss and less corneal edema.1-3,5-7

60 mL/min aspiration rate
University of Texas Southwestern Confocal Microscopy in vivo animal study (Adapted by Alcon; representative CMTF reconstructions)
*Compared to HaNA solutions alone.


Viscoat®


DuoVisc®


DisCoVisc®
Versatile Performance with DuoVisc® Viscoelastic System8

DuoVisc®, as a combination of cohesive and dispersive products, meets each of your procedural needs and preferences to allow a variety of surgical techniques. Following the proven methodology helps to maximise the advantages and minimises the disadvantages of both products.8
Range of Volumes to Cover Each Step of the Procedure
The required amount of OVD depends on a variety of factors, including the procedure, technique and surgeons’ preference. With this in mind, Alcon OVDs are available in several volumes to fit your needs – even more than 1mL.


Viscoat® 0.50 ml
ProVisc® 0.85ml


Viscoat® 0.50 ml
ProVisc® 0.55ml


Viscoat® 0.50 ml
ProVisc® 0.45ml


DisCoVisc® 1 ml
Alcon OVDs are Consistent and Trusted by Surgeons9,10
Global trust in OVD quality is confirmed by Alcon’s leading market position and surgeon experience. Alcon leads the OVD market with over half of the global revenue share.9,10






Instructions for Use (IFU)
For a full list of indications, contraindications and warnings, please visit ifu.alcon.com and refer to the relevant product’s instructions for use.
Alcon Experience Academy
For relevant training content from industry thought leaders
References:
1. Glasser DB, Katz HR, Boyd JE, Shobe SL, Peiffer RL. Protective effects of viscous solutions in phacoemulsification and traumatic lens implantation. Arch Ophthalmol.1989;107(7):1047-1051.
2. Petroll WM, Jafari M, Lane SS, Jester JV, Cavanagh HD. Quantitative assessment of ophthalmic viscosurgical retention using in vivo confocal microscopy. J Cataract Refract Surg. 2005;31(12):2363-2368.
3. Poyer JF, Chan KY, Arshinoff SA. New method to measure the retention of viscoelastic agents on rabbit corneal endothelial cell line after irrigation and aaspiration. J Cataract Refract Surg. 1998;24(1):84-90.
4. Lehmann R, Brint S, Stewart R, et al. Clinical comparison of ProVisc® and Healon in cataract surgery. J Cataract Refract Surg. 1995;21(5):543-547.12.
5. Papaconstantinou D, Karmiris T, Diagourtas A, Koutsandrea C, Georgalas I. Clinical trial evaluating Viscoat® and Visthesia ophthalmic viscosurgical devices in corneal endothelial loss after cataract extraction and intraocular lens implantation. Cutan Ocul Toxicol. 2014;33(3):173-180.
6. Koch D. et al. A comparison of corneal endothelial changes after use of Healon or Viscoat during phacoemulsification. Am J Ophthalmol. 1993;115(2):188-201.
7. Moschos MM, Chatziralli IP, Sergentanis TN. Viscoat versus Visthesia during phacoemulsification cataract surgery: corneal and foveal changes. BMC Ophthalmol. 2011 Apr 29;11:9. doi: 10.1186/1471-2415-11-9.
8. Arshinoff SA. Dispersive-cohesive viscoelastic soft shell technique. J Cataract Refract Surg. 1999 Feb;25(2):167-73. doi: 10.1016/s0886-3350(99)80121-7.
9. Alcon data on file, REF-14204, 2021.
10. DisCoVisc® Direction for Use.
11. Alcon Internal Sales Data.
Please refer to the relevant product direction for use for list of indications, contraindications and warnings.
DUOVISC Viscoelastic System GD72827468717
Discovisc Ophthalmic Viscosurgical Device GD74138174717
VISCOAT Ophthalmic Viscosurgical Device GD64236468117
Registered under Act 737
The content is intended for Healthcare Professionals only, not for general public

