Shockingly Brilliant Distance Vision, Time After Time1-5
Clareon® Monofocal IOLs give your patients functional intermediate vision while maintaining exceptional sharp, crisp distance vision.6-9 But what they see might surprise them.
Shockingly Brilliant Distance Vision, Time After Time1-5
Clareon® Monofocal IOLs give your patients functional intermediate vision while maintaining exceptional sharp, crisp distance vision.6-9 But what they see might surprise them.
Clareon® Delivers Clear, Predictable Performance1-3,10,11*†
- Excellent Quality of Vision with Predictable Functional Intermediate3,12,13
- Maximum Refractive Predictability3,5,14
- Unsurpassed Clarity10*†

*Compared in vitro with TECNIS‡ OptiBlue‡ ZCB00V, TECNIS‡ ZCB00, Vivinex‡ XY-1, Eternity‡ Natural Uni W-60 and enVista‡ MX60. (Surface haze and SSNGs: n=10 lenses per group, P<0.001; Glistenings: n=30 IOLs per group, P<0.001).
†Among the lowest levels of surface haze, SSNGs, and glistenings of competitive IOLs.
‡Trademarks are the property of their respective owners.
Excellent quality of vision with predictable functional intermediate3,6-8,12,13,15
Clareon® IOL delivers functional intermediate vision up to 20/32 at 66 cm while maintaining 20/20 vision at distance6-8,15*†

*Based on two Vivity® registration studies, which used SN60WF as the control, AcrySof® IQ monofocal IOLs provided mean binocular distance corrected intermediate VA at 0.2 logMAR
†Based on Studies with AcrySof®; Clareon® and AcrySof® are optically equivalent with the same -0.2µm aspheric design resulting in improved depth of focus.
UCIVA = Uncorrected Intermediate Visual Acuity DCIVA = Distance Corrected Intermediate Visual Acuity OUS = Outside United States
Clareon® proprietary edge curvature significantly reduces edge glare3,12,13

Clareon® IOL Precision Edge Design

Straight Edge IOL
*Evaluated in a schematic model eye and in vitro evaluation of positive dysphotopsia or glare types photic phenomena. Optical ray trace simulations of incoming light were generated based on a collimated light source with a wavelength of 550 nm for various off-axis angles of illumination (n=5 IOLs per group, +25.0 D). The simulation analyses were verified using a laboratory glare bench-top measurement system, whereby glare components formed from off-axis illumination of IOLs fitted into an artificial eye model were measured. Only clinical studies can confirm whether the differences observed between the IOLs in vitro are clinically significant.
Visualize how glare can impact everyday life with this glare simulation
UKIE-CLA-2100001
Clareon® features a fully usable 6 mm aspheric optic dedicated to sharp, crisp vision3,12,13


Clareon®
Fully usable 6 mm aspheric optic


TECNIS* 1-Piece
Usable aspheric optic limited to 4.9 mm


enVista*
Usable aspheric optic limited to 4.9 mm
Compared with TECNIS* 1-Piece and enVista* IOL in an in vitro study. Images were captured using a Keyence video microscope under precisely controlled conditions. Each IOL (+25.0 D) was placed into a wet cell model eye containing BSS® and a spherical aberration neutral simulated cornea. For each IOL, the target image was captured using the identical test conditions and camera settings without additional focusing. All elements (the Keyence microscope, the IOL in wet cell, the model cornea, and the target image) were located in exactly the same place using the exact same imaging settings on the Keyence in the manual mode. Optics are presented in black and white for illustrative purposes only.
*Trademarks are the property of their respective owners.
Clareon® Guards Against PCO for Long-term Visual Quality3,16

Continuous Posterior Barrier and Low Nd:YAG Rates
Maximum Refractive Predictability3-5,14
STABLEFORCE® Haptics help Clareon® deliver superior axial stability compared to other IOLs3-5,14*†
Variations in the capsular bag size and IOL haptic design lead to IOL axial displacement that can result in residual refractive errors.4

GB-CLM-2300002
*Compared in vitro with enVista† MX60, TECNIS† ZCB00, and Vivinex† XY-1 (n=10 lenses per group, P<0.05).
†Axial displacement results as a function of compression diameter with corresponding simulated dioptric power shift at the corneal plane in vitro.
Clareon® delivers consistent centration across capsule sizes3,5,14*
STABLEFORCE® Haptics provide excellent centration and stability for a range of capsule sizes.3,5,14


GB-CLM-2300003
For illustrative purposes only.
*Compared in vitro with enVista† MX60, TECNIS† ZCB00, and Vivinex† XY-1 (n=10 lenses per group, P<0.05).
†Trademarks are the property of their respective owners.
Advanced Manufacturing Process Delivers Unsurpassed Clarity2,10,18,19
UKIE-CLI-2100002
Clareon® offers long lasting, glistening-free results2,11*
In long-term clinical studies, Clareon® IOLs were reported as glistening-free over 3 and 9 years.2,11*†

*Defined as Miyata grade 0, <25mv/mm2 over 3 years (n=138), and over 9 years (n=20), respectively.
†Compared in vitro with TECNIS‡ OptiBlue‡ ZCB00V, TECNIS‡ ZCB00, Vivinex‡ XY-1, Eternity‡ Natural Uni W-60, and enVista‡ MX60. (Surface haze and SSNGs: n=10 lenses per group, P<0.001; glistenings: n=30 IOLs per group, P<0.001.)
‡Trademarks are the property of their respective owners.
Significantly less glistenings than other IOLs10
In an in vitro study, Clareon® IOL demonstrated lower levels of glistenings compared with TECNIS† and Vivinex† IOLs.10‡

*Compared in vitro with TECNIS† OptiBlue† ZCB00V, TECNIS† ZCB00, Vivinex† XY-1, Eternity† Natural Uni W-60, and enVista† MX60. (Surface haze and SSNGs: n=10 lenses per group, P<0.001; glistenings: n=30 IOLs per group, P<0.001.)
†Trademarks are the property of their respective owners.
‡Denotes statistical significance for comparison of slit lamp surface haze as determined by a one-way ANOVA (P<0.001) compared with Clareon®.
A Rotationally Stable IOL with an Advanced Precision Edge Design and Unsurpassed Clarity5,10,13,20,21
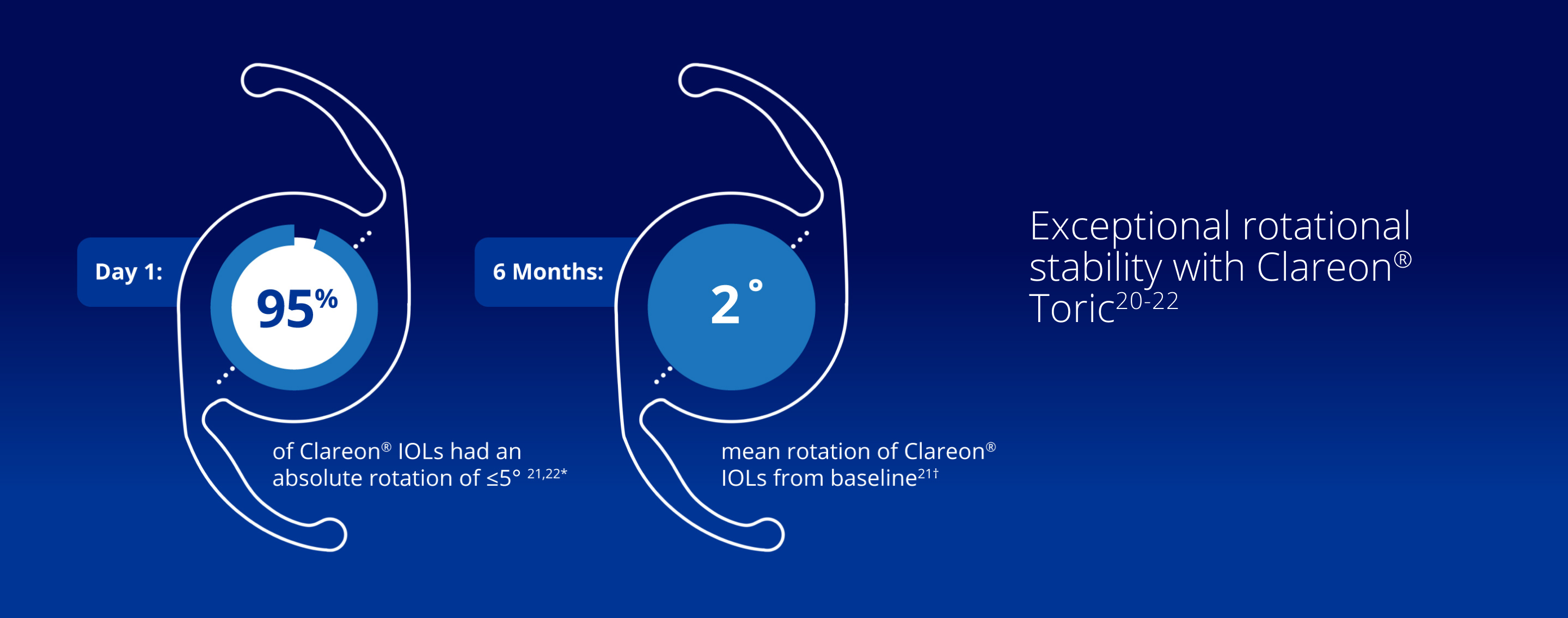
*In an additional analysis, 98.4% of Clareon® IOLs had an absolute rotation of ≤10° between surgery and 6 months.
†Baseline is defined as ≤1-hour post-surgery in a randomized controlled trial (N=141).
Alcon Online Toric IOL Calculator features the Barrett Toric and Holladay Total SIA Toric Algorithms

Available in manual and preloaded delivery devices, specifically designed for the Clareon® platform
Please contact your local Alcon representative for IOL delivery systems
available in your market.
Available in manual and preloaded delivery devices, specifically designed for the Clareon® platform
Please contact your local Alcon representative for IOL delivery systems available in your market.
Clareon® and Clareon® Toric Clinical Studies
Technical Specifications


†Blue Light + UV Filter
‡UV Filter Only

*Based on an average pseudophakic human eye using an SRK/T optical A-constant of 119.1
†Blue Light + UV Filter
‡UV Filter Only
Instructions for Use (IFU)
For a full list of indications, contraindications and warnings, please visit ifu.alcon.com and refer to the relevant product’s instructions for use.
Alcon Experience Academy
For relevant training content from industry thought leaders
References:
1. Lehmann R, Maxwell A, Lubeck DM, Fong R, Walters TR, Fakadej A. Effectiveness and Safety of the Clareon Monofocal Intraocular Lens: Outcomes from a 12-Month Single-Arm Clinical Study in a Large Sample. Clin Ophthalmol. 2021;15:1647-1657. Published 2021 Apr 20. doi:10.2147/OPTH.S295008
2. Oshika T, Fujita Y, Inamura M, Miyata K. Mid-term and long-term clinical assessments of a new 1-piece hydrophobic acrylic IOL with hydroxyethyl methacrylate. J Cataract Refract Surg. 2020;46(5):682-687.
3. Clareon® IOL Directions for Use.
4. Lane, S, Collins, S, Das, K et al. Evaluation of intraocular lens mechanical stability. J Cataract Refract Surg. 2019;45(4):501-506.
5. Alcon Data on File, 2017. [TDOC-0054028]
6. AcrySof® IQ Vivity™ IOL Directions for Use
7. Bala C, Poyales F, Guarro M, Mesa RR, Mearza A, Varma DK, Jasti S, Lemp-Hull J. Multi-country clinical outcomes of a new nondiffractive presbyopia-correcting intraocular lens. J Cataract Refract Surg. 2021 Jun 11. doi: 10.1097/j.jcrs.0000000000000712. Epub ahead of print. PMID: 34288635.
8. Alcon Data on File, 2021. [A01970-REP-211731]
9. Varma, Devesh, et al. Clinical Outcomes of a New Non-Diffractive Presbyopia-Correcting Intraocular Lens From Two Large Confirmatory Studies. American Academy of Ophthalmology. Abstract: PA005.
10. Werner L, Thatthamla I, Ong M, et al. Evaluation of clarity characteristics in a new hydrophobic acrylic IOL in comparison to commercially available IOLs. J Cataract Refract Surg. 2019;45(10):1490-1497. doi:10.1016/j.jcrs.2019.05.017
11. Maxwell A, Suryakumar R. Long-term effectiveness and safety of a three-piece acrylic hydrophobic intraocular lens modified with hydroxyethyl-methacrylate: an open-label, 3-year follow-up study. Clin Ophthalmol. 2018;12:2031–2037.
12. Alcon Data on File, 2017. [TDOC-0050244]
13. Das KK, Werner L, Collins S, Hong X. In vitro and schematic model eye assessment of glare or positive dysphotopsia-type photic phenomena: Comparison of a new material IOL to other monofocal IOLs. J Cataract Refract Surg. 2019;45(2):219-227.
14. Alcon Data on File, 2017. [TDOC-0053564]
15. Alcon Data on File; TDOC – 055575; 09/Apr/2019
16. AcrySof® IQ IOL Directions for Use
17. Alcon Data on File, 2017. [TDOC-0054422]
18. Alcon Data on File, 2020. [TDOC-0057291]
19. Stanojcic N, O’Brart D, Hull C, et al. Visual and refractive outcomes and glistenings occurrence after implantation of 2 hydrophobic acrylic aspheric monofocal IOLs. J Cataract Refract Surg. 2020;46(7):986-994.
20. Lee BS, Chang DF. Comparison of the rotational stability of two toric intraocular lenses in 1273 consecutive eyes. Ophthalmology. 2018;125(9):1325-1331
21. Clareon® Toric IOL Directions for Use.
22. Alcon Data on File, 2019. [TDOC-0055470]
Please refer to the relevant product direction for use for list of indications, contraindications and warnings. Find at: https://ifu.alcon.com

