Clareon® Monofocal IOL
Exceptional clarity that lasts1*
Clareon® Collection IOLs
More options for more patients: four optic designs, one glistening-free* material1,2
Clareon® Monofocal IOLs provide exceptional clarity†, allowing you to confidently deliver the long-lasting refractive outcomes that your monofocal patients expect.1,2
Exceptional Clarity that Lasts1*
Delivers among the lowest levels of glistenings, SSNGs, and surface haze.1,3,4* In long-term clinical studies, Clareon® IOLs were reported as glistening-free over 3 and 9 years.1,3,4*

Proprietary Precision Edge Design5,6
Proprietary edge curvature designed to help reduce PCO and edge glare5,6

Superior Axial Stability7,8
Fibronectin helps bind the material to the capsule, anchoring the lens in place to help reduce PCO and improve stability.6-9


Optical Design with Proven Performance10
Clareon® and AcrySof® share the same -0.2µm aspheric design resulting in improved depth of focus with a fully usable 6mm optic.10††
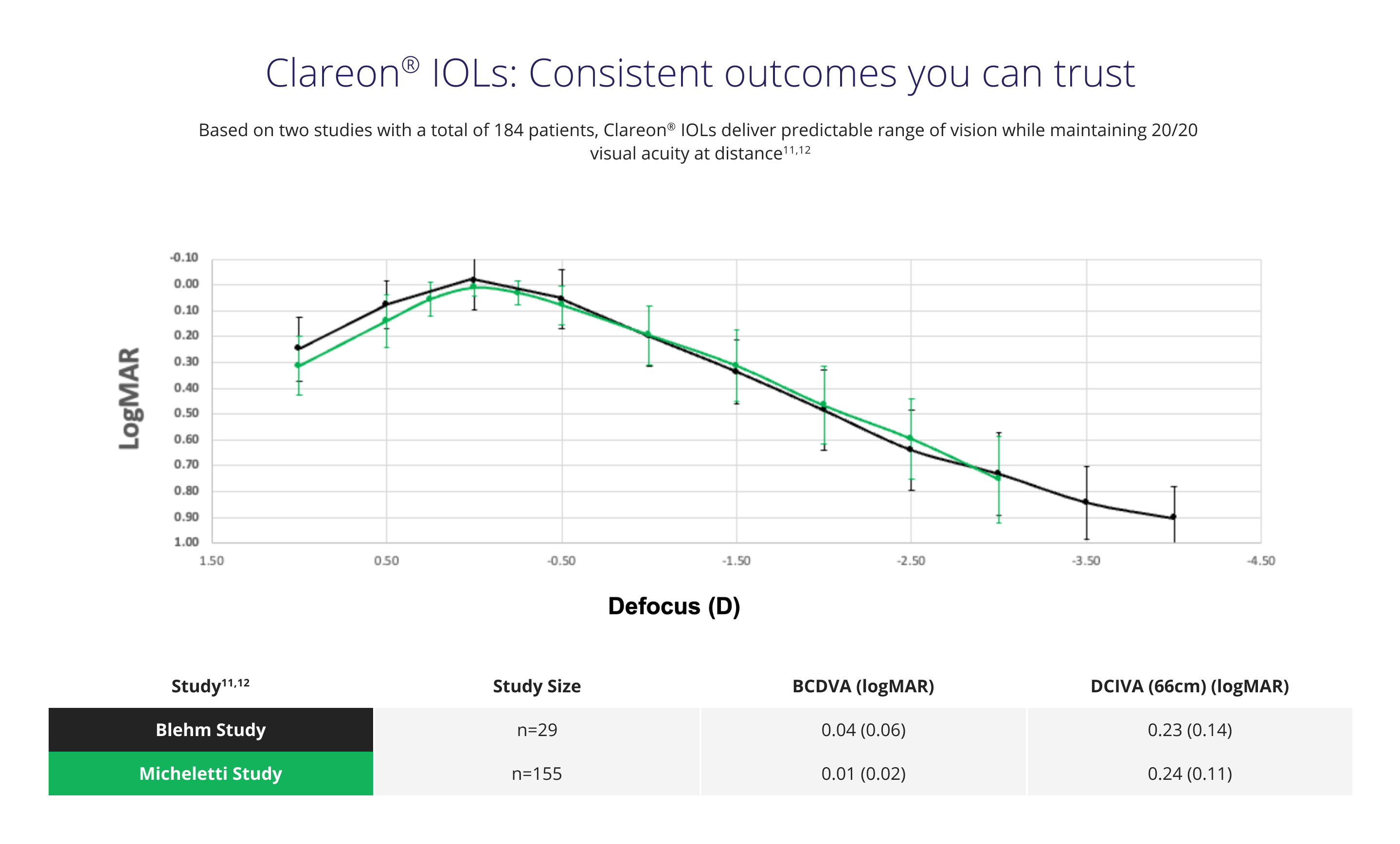
See the difference CLAREON® can make
New advanced manufacturing process combines a proprietary precision edge design with the exceptional BioOptics and BioMechanics of all Alcon IOLs.5,6
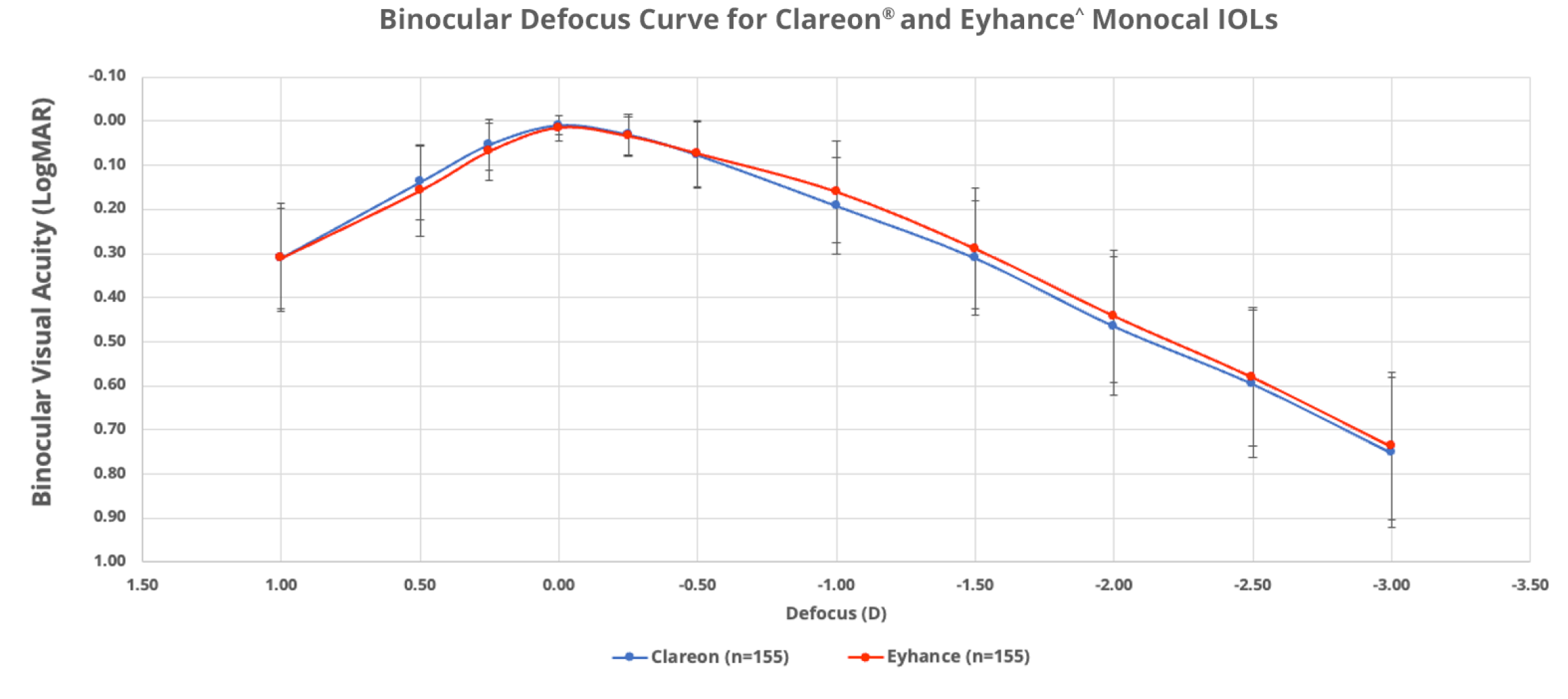
No significant difference in range of vision was observed between Clareon® and Eyhance‡ monofocals
In a clinical study of 310 patients (155 per group), Clareon® and Eyhance‡ IOLs offered comparable binocular range of vision11§
Explore additional resources for the Clareon® Monofocal IOL
The Clareon® collection provides more options for more patients

Clareon® Toric IOL
Manage astigmatism with outstanding axial and rotational stability.7,8

Clareon® PanOptix® IOL
The first and only trifocal lens in the United States, offering a full range of vision and exceptional clarity.1,13†


Clareon® Vivity® IOL
The first and only non-diffractive presbyopia-mitigating technology with exceptional clarity.1,14†
Share our lens selection guide with your patients to help educate them on their Clareon® collection options


Available on Clareon®: Alcon’s most advanced IOL biomaterial to date



* Defined as modified Miyata grade 0, <25mv /mm2 over 3 years (n=138 ), and over 9 years (n=20 ), respectively.
† Based on in vitro examinations of glistenings, surface haze and SSNGs.
†† Clareon® and AcrySof® share the same -0.2μm aspheric design resulting in improved depth of focus.
‡ Trademarks are the property of their respective owners.
IMPORTANT PRODUCT INFORMATION - CLAREON® ASPHERIC FAMILY OF HYDROPHOBIC ACRYLIC IOLS
CAUTION: Federal law restricts this device to sale by or on the order of a physician.
INDICATIONS: The Clareon® Aspheric Hydrophobic Acrylic IOLs include the Clareon® Aspheric and Clareon® Aspheric Toric IOLs and are indicated for primary implantation in the capsular bag in the posterior chamber of the eye for the visual correction of aphakia in adult patients in whom a cataractous lens has been removed. In addition, the Clareon® Aspheric Toric IOL is indicated to correct pre-existing corneal astigmatism.
WARNINGS / PRECAUTIONS: The Clareon® IOL is intended for implantation in the capsular bag only. Physicians considering lens implantation under any of the following circumstances should weigh the potential risk/benefit ratio: Patients in whom the posterior capsule is ruptured, zonules are damaged, or primary posterior capsulotomy is planned. Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/benefit ratio before implanting the IOL in a patient with any of the conditions described in the Directions for Use. For the Clareon® Aspheric Toric IOLs, rotation can reduce astigmatic correction; if necessary lens repositioning should occur as early as possible prior to lens encapsulation. As with any surgical procedure, there is risk involved. Potential complications accompanying cataract and/or IOL implantation surgery may include, but are not limited to, the following: lens epithelial cell on- growth, corneal endothelial cell damage, infection (endophthalmitis), toxic anterior segment syndrome (TASS), retinal detachment, vitritis, cystoid macular edema, corneal edema, pupillary block, cyclitic membrane, iris prolapse, hypopyon, anterior uveitis, hyphema, pigment dispersion, posterior capsule opacification, transient or persistent glaucoma, and secondary surgical interventions. Secondary surgical interventions include, but are not limited to: lens repositioning, lens replacement, vitreous aspiration or iridectomy for pupillary block, wound leak repair, and retinal detachment repair. Prior to surgery, prospective patients should be informed of the possible risks and benefits associated with this IOL as well as the risks and benefits associated with cataract surgery. After surgery, physicians should provide an implant card to patients regarding the IOL implanted. DO NOT re-sterilize the Clareon® IOL by any method. The device is for single use only.
ATTENTION: Reference the Directions for Use labeling for each IOL for a complete listing of indications, warnings and precautions.
REFERENCES:
- Werner L, Thatthamla I, Ong M, et al. Evaluation of clarity characteristics in a new hydrophobic acrylic IOL. J Cataract Refract Surg. 2019;45:1490-1497.
- Lehmann R, Maxwell A, Lubeck DM, Fong R, Walters TR, Fakadej A. Effectiveness and Safety of the Clareon® Monofocal Intraocular Lens: Outcomes from a 12-Month Single-Arm Clinical Study in a Large Sample. Clin Ophthalmol. 2021;15:1647-1657. Published 2021 Apr 20.
- Oshika T, Fujita Y, Inamura M, Miyata K. Mid-term and long-term clinical assessments of a new 1-piece hydrophobic acrylic IOL with hydroxyethyl meth-acrylate. J Cataract Refract Surg. 2020 May;46(5):682-687.
- Maxwell A, Suryakumar R. Long-term effectiveness and safety of a three-piece acrylic hydrophobic intraocular lens modified with hydroxyethyl-methacrylate: an open-label, 3-year follow-up study. Clin Ophthalmol. 2018;12:2031-2037.
- Clareon® IOL Directions for Use.
- Das KK, Werner L, Collins S, Hong X. In vitro and schematic model eye assessment of glare or positive dysphotopsia-type photic phenomena: Comparison of a new material IOL to other monofocal IOLs. J Cataract Refract Surg. 2019;45(2):219-227.
- Alcon Data on File, 2017.
- Lane S, Collins S, Das KK, Maass S, Thatthamla I, Schatz H, Van Noy S, Jain R. Evaluation of intraocular lens mechanical stability. J Cataract Refract Surg. 2019 Apr; 45(4):501-506.
- Alcon data on file, 2017.
- Alcon data on file, 2017.
- Alcon data on file, 2023.
- Alcon data on file, 2023.
- Clareon® PanOptix® Trifocal Hydrophobic Acrylic IOL Model CNWTT0 2021 Directions for Use.
- Clareon® Vivity® Extended Vision Hydrophobic IOL (CNWET0) Directions for Use – USA.



